Home / Products / Article Directory / The use of the medicinal product Altabor for the prevention of influenza and acute respiratory viral infections
Acute respiratory viral infections (ARVI) are leaders among the causes of diseases and temporary disability in young people. Even during an interepidemic period, more than 1/6 of the world population are suffering from those diseases. In Ukraine, over 10-14 million people suffer from acute respiratory viral infections (ARVI) each year, which is 25-30% of the diseases and 75-90% of infectious diseases in our country [7]. According to the National Centre for Epidemiology and Population Health, ANU, ARVIs are a leading cause of morbidity in all countries, and a major cause of premature death in countries where mortality is high [10]. These infections are a leading cause of medical consultation and hospital admission in children, and are the reason for huge sales of over-the-counter and prescribed medical products [11, 12]. Adults fall ill with ARVI about 2-3 times per year; children experience 5-8 infections per year during their first years of life. At least 3.9 million children die annually in the developing world from the serious consequences of these infections (WHO, 2002). 70-80 % of ARVI-related deaths are caused by pneumonia. Acute episodes of lower respiratory tract infections occur ten to fifty times more frequently in developing countries where susceptible individuals become superinfected by bacteria, taking into account poor hygiene and nutrition conditions, low rates of breastfeeding, low birth weights, and smoker families. [10].
Despite the fact that the rates of ARVI-related deaths have significantly declined in developed countries, the rates of primary attacks are not greatly different between developed and developing countries. Moreover, these rates are growing due to urbanization and migration. Viruses causing acute respiratory diseases are identical in both developed and developing countries. Today, there are more than 200 ARVI pathogens [2], including, first of all, influenza virus causing about 20% of all viral infections, followed by parainfluenza, rhinovirus, adenovirus, coronavirus, and respiratory syncytial infections, "SARS" (atypical pneumonia), reovirus infection and enteroviral disease. All ARVI pathogens having similar pathogenetic mechanisms, are highly contagious because they are transmitted by air, affecting the upper respiratory tract. Therefore, in the opinion of S.L. Rybalko et al. [9], medical products for the treatment of ARVIs should have the following pharmacodynamic properties:
• direct antiviral action at all stages of viral infection;
• direct antiviral broad-spectrum action (acting on RNA and DNA of viruses);
• inhibitory action on the influenza virus neuraminidase;
• good bioavailability, so that it could be well absorbed by the upper respiratory tract mucosa;
• detoxification and antioxidant properties;
• immunotropic action without developing refractoriness of immune cells.
According to Order of MoH of Ukraine No. 590 of 12.08.2009 “On Approval of the Guidance "Principles of the Diagnosis and Treatment of Patients with Acute Respiratory Viral Infections" [6], the standard treatment of ARVI patients with chronic nonspecific pulmonary diseases (COPD), diabetes mellitus, and those with chronic infection foci includes ascorbic acid, rutin, second and third generation antihistamines, secretolytics, antibiotics (macrolides, fluoroquinolones, cephalosporins, protected penicillins), and antiviral medicine. According to the recommendations of WHO, 2002, antiviral, immunomodulating and symptomatic medications are recommended for the treatment of ARVIs. The symptomatic therapy of ARVIs includes analgesics/antipyretics because the primary symptom of ARVIs, in particular, of influenza, is fever. The most common antipyretic medicine is paracetamol, which is highly effective and safe,, in particular in children [4, 5].
Unfortunately, in our country as in other CIS countries, systemic antibiotics are the most widely used medicine to treat children with acute respiratory infections, and they are often used to treat viral respiratory infections. According to a study conducted simultaneously in five regions of Russia [13], children had the following ARVIs: 78.4% of children had common cold, 9.3% - acute bronchitis, 8% - tonsillitis, and 3.7% - pneumonia. Antibiotic therapy was given to 43% of children with common cold, 78% of patients with acute bronchitis, 99.2% of patients with acute tonsillitis and 100% of patients with pneumonia. The term "common cold" or “influenza” is a fairly common concept in the Western Europe, the USA, etc. It includes a number of symptoms common for all ARVIs, such as fatigue, throat irritation, runny nose, and fever. The concept of "cold" means that the ARI has a viral origin. That is why the use of antibiotics in half of these patients is at least inexpedient.
Taking into account the ARVI incidence, high contagiousness, risk of serious complications, mortality, and difficulties in choosing appropriate therapy, the prevention of viral upper respiratory tract infections is becoming especially urgent. There are the following types of prophylaxis [1]:
• nonspecific pharmacoprophylaxis involves the use of immunotropic plant medications (adaptogens) and microbially-derived immunomodulators and their synthetic analogues;
• antiviral chemotherapeutic medications during the high incidence of influenza;
• specific prophylaxis - vaccination.
According to the standards of Ukraine, ARVIs should be prevented by means of vaccination and chemoprophylaxis. Therefore, these types of prevention should be reviewed in detail. Vaccination should be provided 2-3 months before an anticipated onset of epidemic caused by a vaccine strain. The effectiveness of vaccine depends upon whether or not the strain of seasonal influenza was predicted properly, so that the vaccine should correspond to the strain, and upon the percentage of vaccinated people. Additionally, Influenza viruses are extremely changeable [8, 9]. Vaccines are commonly chosen on the basis of maximum similarity to the prevailing strains. This is because there is a partial cross-immunity between serotypes of the same subtype. Usually vaccination is effective in middle-aged and young people, thus, 60-90% of vaccinated people form a protective antibody titre. Vaccination effectiveness is much lower in children and elderly people, thus, a protective antibody titre is formed in only 30-60% of the vaccinated population. Moreover, the antibodies formed could not fully correspond to the antigens of current circulating strains, therefore, usually vaccine efficacy is even 20-30% lower. Even those who have a protective antibody titre against current circulating strains, may fall ill with influenza, but the disease may be mild. Thus, the vaccine does not guarantee full protection against influenza. [7].
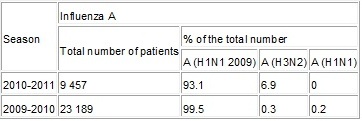
Regarding the chemoprophylaxis, there is a number of unresolved issues. During the 2009-2010 epidemic season, an influenza pandemic was announced due to the prevalence of influenza A (H1N1 2009), influenza B, and seasonal influenza A (H3N2). The situation changed in 2010-2011 (Tables 1, 2) [14].
Table 1
The incidence of influenza in Europe in 2009-2010 and 2010-2011 per 100 000 people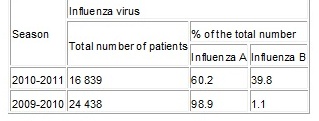
Table 2
The prevalence of influenza A strains in Europe in 2009-2010 and 2010-2011, per 100 000 people
The use of well known antiviral products for the prevention and treatment of influenza had some drawbacks.
In 2009-2010, 97% of patients with influenza A (H1N1 2009) and 100% of patients with influenza B were sensitive to oseltamivir and zanamivir (neuraminidase inhibitors). Total 3526 patients were tested for sensitivity to these drugs. The H275Y neuraminidase mutations of 109 (3.1%) viruses A (H1N1 2009) were reported. This virus became resistant to these medicinal products. All viruses of influenza A (H1N1 2009) and A (H3N2) were resistant to adamantanes [14]. In addition, the high cost, numerous gastrointestinal side effects, narrow spectrum of action of chemically synthesized antiviral medications have raised debates regarding the use of these medications for the prevention of influenza and other ARVIs.
A multicentre open post-marketing study of Altabor tablets 20 mg, manufactured by PJSC SIC “Borshchahivskiy Chemical Pharmaceutical Plant” for the prevention of influenza and acute respiratory infections was conducted in Ukraine during the period of the influenza pandemic (December 2009 - February 2010),.
According to the Ministry of Health of Ukraine, the increased incidence of influenza and ARVIs within the period from December 2009 to February 2010 was reported in ten control cities (Vinnitsa, Dnipropetrovsk, Donetsk, Zaporizhia, Kyiv, Odessa, Simferopol, Kharkiv, Chernihiv) and was mainly due to the circulation of influenza virus A (H1N1) among the population. Taking into account that during the study there was an unfavorable epidemiological situation, the study of the effectiveness of Altabor used for the prevention of ARVIs and influenza was relevant, and this situation significantly enhanced the value of the results obtained.
As opposed to synthetic antivirals, Altabor is a plant product. The active substance of the product is dry extract of infructescences of grey alder and black alder. It contains a mixture of oligomeric ellagitannins (glycosides on the basis of phenolic acids: ellagic, trioxybenzoic, and dilacton valonic acids). According to an experimental study of the substance and dosage form of Altabor, the polyphenolic compounds of the extract has pronounced antiviral properties against several viruses of influenza, vesicular stomatitis and herpes simplex. The mechanism of antiviral action of Altabor is the induction of interferon synthesis, inhibition of neuraminidase of influenza virus and herpes virus-specific thymidine kinase. This leads to inhibition of the viral DNA synthesis and reduction in the infectious period. The additional pharmacological properties of Altabor are as follows: a broad-spectrum antibacterial action against gram-positive (Staphylococcus aureus, Bacillus subtilis) and gram-negative (Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Klebsiella) microorganisms, pronounced antioxidant, anti-inflammatory, membrane-stabilizing, and analgesic effects.
Study objectives
The objective of this study was investigation of effectiveness and tolerability of Altabor tablets 20 mg when used for the prevention of influenza and ARVIs, and their complications.
Study design
The study included 200 patients (124 women (62%) and 76 men (38%)) aged from 18 to 65 years (average age was 38.84±0.87 years), who were given Altabor at a dose of 40 mg (2 tablets) 3 times a day during one week.
The study was conducted in 5 hospitals (3 of them are located in Kharkiv and 2 - in Kyiv). That is why all the patients were divided into 5 groups. The main inclusion criterion was the absence of influenza symptoms or any ARVIs and their complications at the beginning of the study. When the effectiveness was analyzed, however, 13 subjects were excluded from the study based on this criterion (some symptoms of ARVIs were identified), but they were included in the analysis of tolerability, because this factor did not affect this parameter. Therefore, the effectiveness was evaluated in 187 patients; the tolerability was evaluated in 200 subjects.
Exclusion criteria:
• known hypersensitivity to the components of the product,
• aggravated allergic anamnesis,
• pregnancy, breastfeeding,
• concomitant decompensated disease or acute conditions that could significantly affect the results of the study,
• participation in any other clinical study within thirty days before the study.
36 patients with concomitant diseases, who were not excluded in accordance with the exclusion criteria, took other medicinal products, but not the following products:
• oral and parenteral antiviral agents including immunostimulators;
• systemic antibacterial agents;
• plant-based products.
The patients were examined on the 1st, 8th and 15th study days. The examination included questioning of the volunteers, check-up and laboratory tests (complete blood count) on the 1st and 8th study days. The observations of patients were recorded in their diaries from the 1st to the 7th study days, and the volunteers were interviewed by phone on the 15th study day. Patient’s diary contained the time of administration of Altabor (morning, day, evening), possible clinical symptoms of ARVI/influenza (throat irritation, runny nose, headache, muscle pain), and measurements of heart rate and body temperature. The data on general condition (good, satisfactory, poor) and adverse reactions was also recorded.
There was no control group in this study.
The statistical data analysis has been performed in two stages, based on the data recorded in the case report forms and patients’ diaries. Taking into account that the study was conducted at 5 clinical sites, the homogeneity of the set of patients was evaluated at the first stage of statistical analysis. Then, in order to evaluate the effectiveness and tolerability of the study medicinal product, statistical calculations were performed for each of the five groups.
Efficacy Evaluation Results
Prior to the study, 187 patients included in the efficacy evaluation, did not have ARVI or influenza symptoms, i.e. they did not have any cough, throat irritation, nasal congestion or runny nose. Vesicular breath sound was checked by using auscultation of the lungs. The product was considered effective if on the 8th and 15th study days, none of the listed above symptoms was detected.
Comparison of patients’ diaries
Parameter “General Condition”
McNemar’s test was used to evaluate the effect of the therapy on the parameter “general condition”.
There was no patient with the parameter “general condition” evaluated as “poor” in groups 1, 2 and 5. In group 3, “general condition” of 1 patient (2.5 %) was evaluated as “poor” on the 4th study day. In group 4, only 1 subject (2.5 %) evaluated the “general condition” as “poor” on 4th to 7th study days. This indicated that the tolerability and efficacy of Altabor used for the prevention of ARVI were good.
According to the patients’ diaries, the frequency of “satisfactory” general condition remained practically unchanged in groups 3 and 5 during the 7-day study of the test product. The relative frequencies of “satisfactory condition” remained at the same level, or uncertainly decreased in the other groups during the period of administration of Altabor (Fig. 1).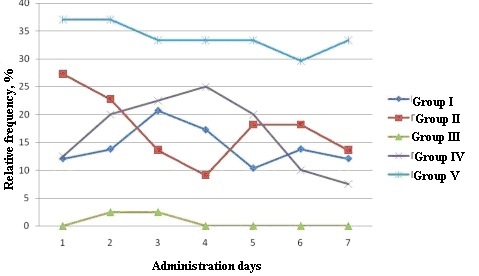
Fig. 1. Relative frequency of “general condition” evaluated as “satisfactory”
Initially the frequency of “good” general condition was significantly higher as compared to the frequency of “satisfactory” and “poor” conditions. The general condition of all the patients in group I did not change on 1st, 8th and 15th days, and was assessed as “good” in 100% of the patients. The number of patients whose general condition was “good” slightly grew in all the study groups after 7 days of administration of Altabor.
Parameter “body temperature”
Only 1 patient in group 1 had changes in the parameter “body temperature”, therefore, the frequency of increase in temperature during the study days was 1.7%, i.e., only 1 patient out of 187 fell ill. The body temperature was normal in 100% of the patients on the 7th study day, which demonstrated the efficacy of Altabor tablets administered during 7 days.
Parameter “throat irritation”
The parameter “throat irritation” changed in 11 out of 187 patients in groups 1, 3 and 4, therefore, the further analysis was carried out for those groups. Most probable, the irritated throat was due to the astringent properties of Altabor.
This symptom was recorded in 1.7% of the patients in group I from study day 2 to study day 7 and 3.4% was recorded on study day 5.
“Throat irritation” was reported in 2.5% of the patients in group 3 from study day 3 to study day 7, and 7.5% was recorded on study day 2.
5% of the subjects in group 4 reported this symptom from study day 3 to study day 5, and only 1 patient (2.5%) had this symptom on study day 6 (Fig. 2).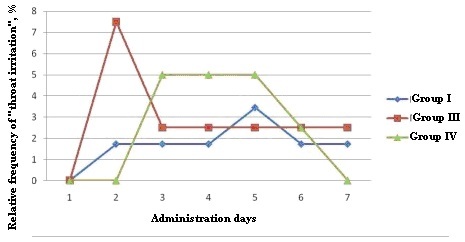
Fig. 2. Relative frequency of ‘throat irritation”
Based on the diagram above, the positive effect of Altabor on the improvement of this parameter in all the patient groups could be noticed on study day 7.
No “throat irritation” was reported on study days 7 and 15, which demonstrated the efficacy of Altabor tablets 20 mg.
Parameter “runny nose”
2 patients in groups 1 and 3 recorded runny nose, therefore, the further analysis was carried out for those groups. 1 subject (1.7%) in group 1 recorded runny nose in his diary from study day 3 to study day 7; runny nose was recorded in the diary of 1 patient (2.5%) in group 3 from study day 4 to study day 7 (Fig. 3).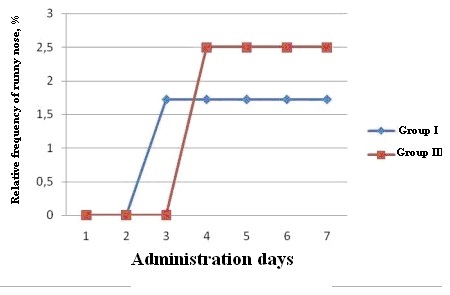
Fig. 3. Relative frequency of “runny nose”
Parameter “headache”
Headache was recorded in the diaries of 18 patients in groups 1, 3 and 4, therefore, the further analysis was carried out for those groups (Fig. 4). It should be noted that headaches were mainly due to concomitant cerebrovascular diseases, high blood pressure, and vertebrogenic disorders.
“Headache” was observed in 1.7% of the patients in group I on study day 2 and from study day 5 to study day 7, and in 3.4% of the patients on study day 3.
This parameter was recorded in the diaries of 2.5% of the subjects in group 3 on study day 1 and from study day 3 to study day 7.
In group 4, “headache” was observed in 2.5% of the patients from study day 2 to study day 6, in 5% of the patients from study day 4 to study day 6, and in 7.5% of the patients on study day 4.
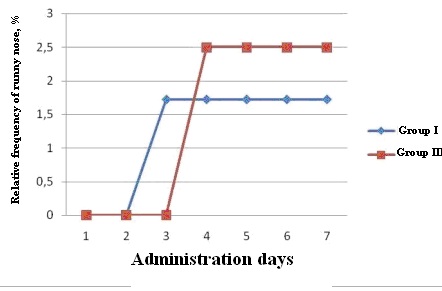
Fig.4. Relative frequency of “headache”
The maximum relative frequency of headaches was 6.9% in group 1, 15% in group 3 and 10% in group 4 (day 4).
After the use of Altabor, the positive changes in the frequencies of headache were observed in all the groups on study day 7.
Parameter “pain in muscles”
Pain in muscles was observed in 1 patient (2.5%) in group 3, therefore, the further analysis was carried out for that group. Positive changes in this parameter were observed on study day 7, which demonstrated efficacy of the test product.
Therefore, the use of Altabor tablets 20 mg lowered the risk of falling ill with ARVIs and influenza in a population group of “high risk”, who are in contact with patients at the time of seasonal increase in morbidity (workers of hospitals and other medical institutions, educational institutions, etc).
Nevertheless, according to the patients’ diaries, the use of Altabor in 2 subjects (1.07%) can be considered ineffective because they observed symptoms of ARVI/influenza up to study day 8.
When Altabor is used for the prevention of influenza and ARVIs, Altabor increases the body's resistance to viral infections and ensures reduction in the incidence of morbidity during an unfavorable epidemiological situation. The incidence of ARVI and influenza was 4% in the Kyiv and Kharkiv regions at the time of the clinical trials, and the morbidity after the prophylactic use of Altabor tablets 20 mg was 1.07%. Thus, the Altabor dose of 2 tablets 3 times a day for 7 days should be considered effective.
According to the results of the physical examination (Day 8) and questioning of the patients by phone (Day 15), there were no cases of influenza/ARVI complications (pneumonia, lung abscess, empyema, sinusitis, otitis media, encephalitis, meningitis, myocarditis, toxic chemical shock). Therefore, taking into account "the incidence of complications" in patients who took a 7-day prophylaxis course of Altabor (evaluated on study days 8 and 15), the medication should be considered effective in 100% of the cases.
Results of Safety Evaluation
The assessment of tolerability included the data obtained from 200 patients taking Altabor.
During this study, 30 events of adverse reactions/adverse events (AR/AEs) were observed in 18 patients. All of them were patients’ complaints: 18 cases of headache, 11 cases of irritation of throat, and 1 case of dizziness.
All the AR/AEs registered on study days 2 to 7 were insignificant. No treatment of AR/AEs was provided; taken actions: none; outcome: recovery without sequelae.
According to the results of this study, the tolerability of the study product was assessed as "good". All the reported events of AR/AEs were non-serious, mild and did not require any treatment. The association of AR/AEs with the administration of the medication in the case of headache and dizziness was assessed as unlikely, and in the case of throat irritation was assessed as probable.
Altabor was positively assessed by other researchers. E.A. Dobra writes [3] that in adults, especially in elderly people, who may have complications after viral diseases, Altabor is highly recommended for the prevention of influenza and ARVIs because it has all the advantages of plant-derived medications. This is polyvalence of action due to biologically active substances of alder, and due to the absence of nephrotoxicity and hepatotoxicity, which are possible when synthetic medications are used [3].
Conclusions
Taking into account the prevalence, complications, mortality, high contagiousness, difficulties in choosing optimal therapy, acute respiratory viral infections are currently leading health problems worldwide. Prevention of ARVI is the best solution to these problems.
Therefore, the use of Altabor 40 mg (2 tablets) 3 times daily for 7 days for the prevention of influenza/ARVIs during influenza and ARVI epidemics has been scientifically justified, because it significantly reduces the incidence of those diseases and the risk of complications within 15 days from the day of taking Altabor.
Altabor, the product manufactured by PJSC SIC "Borshchahivskiy CPP" is effective, safe and well tolerated. The events of irritated throat were rare and occurred due to astringent properties of Altabor.
References
1. S.V. Valyuh, S.Yu. Shtrygol, V.F. Ostashko "Acute Respiratory Viral Infections: Modern Approaches to the Treatment and Prevention" // Pharmacist. - 2008. - No. 1 (on-line)
2. O.I. Hrynevych, V.I. Matyash "Etiopathogenic Prevention and Treatment of Influenza and ARVIs: New Opportunities" // Ukrainian Medical Journal (online) - 2001.- No. 4 (84) VII-VIII (on-line)
3. E.A.Dobra "Antiviral Medicine for the Pharmacotherapy of Influenza and ARVI» // Provizor.- 2011. - No. 2 (on-line)
4. I.A.Zupanets, N.V. Bezdetko, V.A. Usenko "Pharmaceutical Care of Patients with Cold. Symptomatic Treatment of Patients with Increased Body Temperature (Fever) // Provizor. - 2002. - № 11 (on-line)
5. I.A.Zupanets, N.P. Bezuglaya "Modern Approaches to the Effective and Safety Pharmacotherapy of Influenza and ARVIs in Paediatrics" // Modern Paediatrics - 2010.- № 1 (29) - p. 140-144.
6. Ministry of Health of Ukraine. Order of MoH of Ukraine No. 590 of 12.08.2009 “On Approval of the Guidance "Principles of the Diagnosis and Treatment of Patients with Acute Respiratory Viral Infections” // Ukrainian Medical Journal.- 2010.- No. 6(80).- p. 24–29
7. A.M. Pechinka, M.I. Dzeman. "Acute Respiratory Infections: Issues of Clinical Diagnosis and Treatment (lecture)" // Ukrainian Medical Journal.- 2010.- No.5 (79) IX - X (on-line)
8. M.P. Pinchuk "The Role of Immune Disorders in Patients with Influenza and Methods of their Correction" //New Medicine of the Millennium.- 2010.- № 2.- p. 16-23.
9. S.L. Rybalko, Ye. A. Krasnobaev, E.N. Zherebtsova et al. "Current State of the Influenza A H1N1 2009 Issues" // Ukraine. Health of the Nation.- 2010.- No.3 (15) .- p. 169-178.
10. Douglas R.M., D`Souza R.M. "The control of morbidity and mortality from acute respiratory infection." // Suppl. to Health Transition Review. – 1996. – Vol. 6.– p.245-252
11. Leowski, J. "Mortality from acute respiratory infections in children under five years."// World Health Statistics Quarterly.- 1986.- Vol. 39.- p.138-144
12. Pio, A., J. Leowski and H.G. Ten Dam. "The magnitude of the problem of acute res-piratory infections." // Acute Respiratory Infections in Childhood, Proceedings of an International Workshop, Sydney, August, 1984. Adelaide: University of Adelaide. 1985. p. 3-16.
13. Stratchounski L., Ratchini S., Kuznetzov S., et al. "Antimicrobials prescription in outpatient children with acute respiratory tract infections in Russia." // Spanish Journal of Chemotherapy. – 2000. - Vol. 13 (Suppl. 2): 36. - Abstract N M244
14. World Health Organization 2011"Summary of the first post-pandemic influenza season in the first post-pandemic influenza season in the WHO European region: 2010-2011"// www.euro.who.int/_data/assets/pdf_file/0010/153379/flu_2010-2011_summary.pdf
05.09.2013 I.A.Zupanets, Ye.V.Gerasimenko, A.S.Shalamay, T.V.Sayenko

