Home / Products / Article Directory / The Use of Ferumbo in Children with Iron-Deficiency Anaemia
Iron-Deficiency Anaemia (IDA) is a disease caused by iron homeostasis disorders due to insufficient dietary intake and iron malabsorption, and/or iron loss caused by bleeding (intestinal, uterine or urinary tract bleeding), which leads to a decreased haemoglobin level, and, in severe cases, to the low haemoglobin level per ml of blood [1].
Anaemia is most commonly caused by Iron deficiency and affects all population groups worldwide. Iron deficiency (ID) is most common among children, adolescents, pregnant women and women of reproductive age. According to WHO (2001), there are 20% of children aged up to 4 years; and 5.9% of children of 5-14 years old with anaemia in developed countries: these figures are even higher in developing countries, and are 39% and 48.1%, respectively[2]. In Russia, the prevalence of iron deficiency anaemia among children in different regions is from 14 to 32.4% [3]. According to the Ministry of Health (2000), the prevalence of iron deficiency anaemia in children in Ukraine is about 3.6% [4],, which may indicate inadequate diagnosis of this disease.
Iron deficiency is a major problem not only due to its prevalence, but, in the first place, due to the fact that it affects the function of many body systems, thus, affecting various body organs. Iron is not only required for binding to the oxygen and, thus facilitating its transport from the lungs via the arteries to all cells throughout the body, and depositing it by haemoglobin and myoglobin, but iron is also required for the synthesis of enzymes involved in many body biochemical processes: the mitochondrial respiratory chain, citrate cycle, DNA synthesis, metabolism of collagen, tyrosine, and catecholamines. ID leads to impaired functining of various body organs and systems: asthenic syndrome, damaged mucous coat of digestive tract, skin, nails and hair changes, reduction in the level of body nonspecific defence factors, various disorders of cardiovascular system; tachycardia and hypotension, anaemic dysmetabolic myocardiodystrophy [5].
Iron is transported by plasma gamma globulin, transferrin, produced by the liver. Iron bound to transferrin is transferred to body cells, where haemoglobin, myoglobin and certain enzymes are produced. The absorbed iron is deposited as a bound compound ferritin, mainly in the liver. Trivalent iron is necessary to form the haem, which gives an increase in haemoglobin levels [6].
Children may develop IDA due to a number of causes, which can be into-, ante-, intra- and postnatal ones, depending on different periods of child's life. ID could be due to nutritional iron deficiency, gastrointestinal disorders resulting in iron malabsorption, impaired iron transport, intensive growth or blood loss. The lack of iron supply to the bone marrow to red blood progenitor cells, disorders of haemoglobin synthesis, ineffective erythropoiesis, decrease in the erythroid cell pool, reduction in the circulating life of red blood cells due to the damage of lipid components of membranes and lack of antioxidant enzymes may crucially contribute to IDA pathogenesis [7 ].
Ferrous (II) iron- or ferric (III) iron-based products are conventionally used to treat patients with iron deficiency anaemia. Ferrous-iron products are well absorbed, but when used at high doses they may cause damage to the gastric mucosa. Ferric salts are less harmful to the mucous membrane of digestive tract, but are less bioavailable. Ferric iron-based products are currently widely used for the treatment of anaemia. Their efficacy is not lower than the efficacy of ferrous salt-based products and, as opposed to the latter, they cause much fewer side effects in the form of dyspepsia [8].
Recently PJSC SIC "Borshchahivskiy CPP" developed and introduced into production an anti-anaemic product in the form of syrup FERUMBO. The active ingredient of FERUMBO is iron (III) hydroxide polymaltose complex. This product is recommended for the treatment of patients with iron deficiency anaemia caused by prolonged blood loss, and in case of increased need for iron (intensive growth of the adolescents, pregnant women), nutritional iron deficiency, iron malabsorption caused by digestive tract disorders.
FERUMBO has a pronounced anti-anaemic action: stimulates haemo- and erythropoiesis, normalizes haematological parameters, and promotes the restoration of serum iron concentrations to normal physiological concentrations. Iron (III) hydroxide polymaltose complex is stable and does not release free iron ions and, therefore, does not have side effects of iron (II)-based products, which are irritation of digestive tract coats, staining of teeth, and metallic taste in the mouth. When ingested, iron (III) hydroxide polymaltose complex is quickly absorbed from the duodenum and small intestine (the higher degree of iron deficiency, the better it is absorbed). The quick absorption of iron (III) hydroxide polymaltose complex excludes overdosing, which may occur when simple iron (II) salts are absorbed against a concentration gradient. Iron, which is a component of iron (III) hydroxide polymaltose complex, does not have a pro-oxidant property intrinsic to simple iron (II) salts. As FERUMBO contains iron in the form of iron (III) hydroxide polymaltose complex, iron does not form insoluble chelates with food components (phytin, oxalates, tannin) or with medications (tetracyclines, antacids).
A comparative clinical study of the efficacy and safety of the medical products Ferumbo, syrup 50 mg/5 ml, manufactured by PJSC SIC “Borshchahivskiy CPP” and Ferrum Lek, syrup manufactured by the Company Lek, Slovenia, was conducted on the base of the Department of Connective Tissue Disorders in Children of the Institute of Paediatrics, Obstetrics, and Gynaecology of the AMS of Ukraine in 2004. It involved 60 children with IDA (31 girls and 29 boys) aged 2-15 years. Patients of the main group (30 children) took FERUMBO syrup in 100 ml bottles, patients of the control group took the reference syrup. Patients of both groups were selected by age (average age 7.9 + 4.1 years), physical status, concomitant diseases, and they have degree I anaemia (before the treatment, their corpuscular haemoglobin was within 90-110 g/l) .
The patients of the main group received FERUMBO at the following doses: children aged from 2 to 3 years - 1 scoop (5 ml) per day (equivalent to 50 mg of iron); from 3 to 6 years - 1 scoop 1-2 times a day; from 6 to 12 years - 1 scoop 2 times a day; children over 12 years - 2 scoops 2-3 times a day. The medicine was administered 20-30 minutes before meal, and in the case of individual hypersensitivity to iron products - 30 minutes after meal or between meals. The patients of the control group received the reference products in bottles (100 ml) according to the same scheme. The treatment lasted 30 days. Taking into account that the majority of children had certain concomitant diseases (digestive tract disorders, diseases of the respiratory system, nervous system, connective tissue dysplasia, rickets, exudative diathesis, nutritional deficiency, helminthic invasion), medications to treat those diseases were allowed during the study.
Clinical and laboratory parameters were monitored in all the children before administration of iron supplements and after 30 days of the therapy. Subjective complaints (dizziness, weakness, headache, palpitations, fatigue, chest pain, exertional breathlessness) were evaluated in accordance with a score scale: 0 - no signs; 1 – moderate manifestations; 2 - pronounced manifestations. The laboratory examination scheme included a complete blood cell count (haemoglobin, counts of erythrocytes, leukocytes, platelets, leukogram, ESR), urinalysis, and parameters of iron metabolism: serum iron concentration (SI), total iron-binding capacity of serum (TIBC), latent iron-binding capacity of blood serum (LIBC) (determined by the difference between SI and TIBC), transferrin saturation index (TSI) (it was determined as a ratio of trans¬fer-ring bound iron and a TIBC index and expressed in per cent). The findings were proces¬sed according to the method of variation statistics by using Microsoft Excel. The accuracy of the changes in the parameters after the treatment was assessed according to Student's t test [9].
IDA was diagnosed based on not only the haemoglobin level below 110 g/l and laboratory tests demonstrating ID in the body (low SI concentrations, low NTI, increased TIBC and LIBC), but also on the clinical signs of sideropenic anaemic syndrome. Trophic disorders of the skin, nails, hair, mucous membranes; intestinal malabsorption accompanied by dysphagia and dyspepsia; changed taste and sense of smell; autonomic disorders; myalgia and muscle hypotonia; immunity disorders (increased incidence of acute respiratory and intestinal infections) were signs of sideropenic syndrome caused by decreased activity of iron-containing enzymes. It was found that when a significant change of the iron transport pool occur in young children (3-5 years), clinical signs of sideropenia were manifested to a lesser degree than in school children. This demonstrates that the frequency and severity of sideropenic manifestations depend on the duration of ID. All the patients had anaemic syndrome manifested as symptoms caused by circulatory and tissue hypoxia, such as pale skin and mucous membranes, weakness, fatigue, drowsiness, irritability, changes in the cardiovascular system (tachycardia, muffled tones, anaemic systolic murmur, tendency to hypotension, exertional breathlessness).
Almost all patients typically complained about decreased appetite, dyspepsia, fatigue, and headache. The least pronounced subjective symptoms were dizziness, palpitations and chest pain. The most pronounced clinical signs of iron deficiency anaemia in both groups of patients were pallor, dry skin, hyperkeratosis within the knee and elbow joint regions, paleness and atrophy of the mucous membranes, angular stomatitis, and exertional breathlessness. 9 (30%) children in the main group and 7 (23.3%) children in the control group had hypotension. The incidence of tachycardia was the same in both groups: 8 (26.7%) patients.
Clinical efficacy of FERUMBO and the reference product was evaluated in accordance with a conventional score scale. Tolerability was evaluated by a scale based on subjective symptoms and sensations reported by the patients to their doctor, and objective data obtained during the treatment. Additionally, the changes in laboratory parameters were taken into consideration, as well as the incidence and nature of adverse reactions.
After 30 days of administration of iron medicinal products, the patients of both groups showed a significant reduction in the severity of pallor of mucous membranes, fatigue, dizziness, palpitations, heart pain, headache, exertional breathlessness. The majority of the patients also reported improvement in appetite, normalization of sleep, increased mood, reduced emotional lability. There were no significant differences in the dynamics of these clinical parameters in the children of both groups. It should be noted that 3 patients in each group had a short-term (1-3 days) dyspeptic disorders: nausea, bad taste in the mouth, loose stool, which disappeared when patients started to take the product after meals.
The patients of the main and control groups had significant positive changes in laboratory parameters after the treatment (See the Table below).
Table
Comparison of Laboratory Findings in the Patients of Main and Control Groups Before and After the Treatment
Parameter Main Group (FERUMBO) Control Group (reference product)
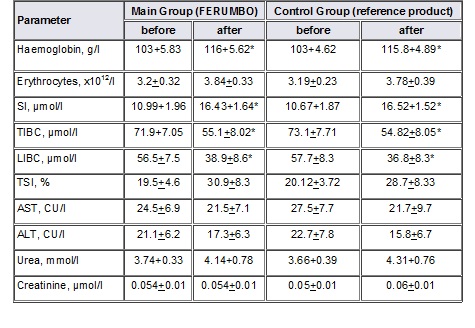
The data above demonstrate that the patients of both groups had pronounced ID before the treatment, which was confirmed by the reduced levels of corpuscular haemoglobin, SI and TSI, increased TIBC, and LIBC. The comparison of those parameters after the treatment with FERUMBO and the reference product revealed that the majority of the children of both groups — 28 (93.3%) children of the main group, and 26 (86.7%) children of the control group, increased levels of corpuscular (haemoglobin), plasma (SI) and transport (TIBC, LIBC) iron pools, and recovery of amounts of iron involved in erythropoiesis (TSI). There were no significant differences between the laboratory parameters of the main and control groups. The serum urea and creatinine, transaminase activity (ALT, AST) were within standard values in all the patients before the administration of FERUMBO. The data analysis of these parameters 30 days after the beginning of the treatment did not revealed any significant differences in the main and control groups and between them, which demonstrated that there were no negative effects of FERUMBO and the reference product on the body metabolism.
It was demonstrated that the efficacy of FERUMBO during the treatment of the children with IDA was high enough (the positive effects were reported in 26 (86.6%) children) and it is not inferior to the effects of the reference product (its positive effect was demonstrated in 27 (90%) children). The tolerance to FERUMBO was considered as “very good” in 27 (90%) and “good” in 3 (10%) patients, which is not inferior to the efficacy of the reference product (26 (86.7%) and 4 (13.3%) patients, respectively). There were no side effects requiring discontinuation of the investigational medicinal products or additional medical care; no individual intolerance of the products was registered.
Therefore, the study results demonstrate a high clinical efficacy of and good tolerance to FERUMBO syrup, which comply with the same of the reference product. When FERUMBO is used at recommended doses, it gives positive effects and improves clinical and laboratory parameters in children with IDA; it is well tolerated. In some cases, an individual regimen of administration of the product may be required. The study findings demonstrated the therapeutic bioequivalence of FERUMBO. By registering and manufacturing this product, PJSC SIC “Borshchahivskiy CPP” greatly contributes to solving the IDA problems in Ukraine.
REFERENCES
1. N.M. Pyasetskaya. Clinical approach to the problem of iron deficiency anaemia in neonatology and paediatrics (lecture for practitioners). - Kyiv, 2004 – 15 p.
2. Iron deficiency anaemia. Assessment, prevention and control/ A guide for programme managers. World Health Organization, 2001: 114 p.
3. Anaemia in children: diagnosis and treatment / Ed. A.G. Rumyantsev, Y.N. Tokarev. - M., 2000 - 128 p.
4. Iron deficiency anaemia: current approaches to diagnosis and treatment / S.N. Gaidukova, S.V. Vydyborets, L.A. Sivak, T.S. Shirinyan - K., 2003 – 32 p.
5. Handbook of Haematology / A.F. Romanov, Ya.I. Vygovska, V.E. Lohynsky etc. .; Eds. A.F. Romanova. - K. Zdorovia, 1997 – p. 46-58.
6. G.V. Arkadieva, Diagnosis and treatment of iron deficiency anaemia. - M., 1999 – 59 p.
7. Iron deficiency and iron deficiency anaemia in children. / Ed. N.S. Kislyak, T.V. Kazakov, N.A. Mazurina. - M., 2001 - 143 p.
8. I.N. Zakharova, A.L. Zaplatnikov, N.E. Malov. The choice of medicine for ferrotherapy of children with iron deficiency anaemia. / Russian Medical Journal, Volume 11, No. 1, 2003.
9. O.P. Mintzer, B.N. Ugarov,, V.V. Vlasov. Methods of processing medical data. - K. Vyscha Schola, 1982 - 159 p.
15.05.2006 L.I. Omelchenko, A.G. Tsypkun, I.V. Dudka, V.B. Nikolaenko, L.A. Datsenko, V.K. Tishchenko, S.K. Stryzhak.
The Institute of Paediatrics, Obstetrics, and Gynaecology of the AMS of Ukraine

